Don’t miss these sessions on how real-world site data, AI insights, and aligned workflows work together to streamline feasibility, strengthen collaboration, and elevate clinical trial execution.
Connect with Advarra at SCOPE
We can’t wait to meet you at SCOPE 2026! Let’s connect in Orlando to talk about new ideas, stronger site partnerships, and the innovations moving clinical research forward.
VISIT BOOTH 701

Discover what’s new
Get a first look at our latest innovations, including site-facing milestone tracking in Study Collaboration for clearer visibility across stakeholders, and Braid, our Data and AI engine that delivers actionable intelligence throughout the trial lifecycle.
Weaving Intelligence into Clinical Trials
Meet Braid™, Advarra’s Data and AI Engine
Powered by the industry’s largest operational dataset, Braid transforms unstructured data into actionable intelligence—enabling smarter trial design, faster decisions, and streamlined execution across the clinical trial lifecycle.
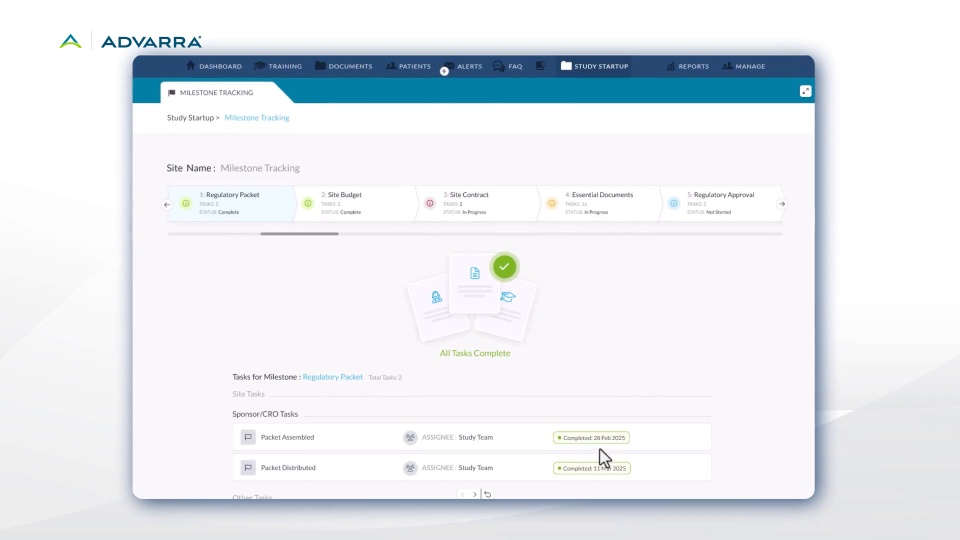
Introducing Site-Facing Milestone Tracking in Advarra Study Collaboration
- New guided site journeys give sites clear visibility into study startup tasks.
- Sponsors and CROs get a real-time, unified view of progress.
- Built with research site input to boost transparency and speed.
- Improves collaboration and reduces startup delays.
Join the Conversation
Site Feasibility of the Future—Site Intelligence from the Source with Syneos Health
Tuesday, February 3 at 11:05 am
Speakers:- Ashley Davidson, Vice President, Product Lead, Advarra
- Tammy D'Lugin-Monroe, Head, Trial Enablement Solutions, Syneos Health
Access to site performance data is transforming how we evaluate and qualify clinical trial sites. By integrating these insights with feasibility workflows, sponsors and CROs can achieve a more holistic, data-driven approach that reduces site burden, accelerates decision-making, and redefines site feasibility for the future.
Designing with Foresight: Turning Operational Data into Protocol Performance with AstraZeneca
Tuesday, February 3 at 3:55 pm
Speakers:- Ian Bailey, Managing Director, AI & Data Science Analytics, Advarra
- Jamie Bendrick-Peart, Senior Director, Innovation and Strategic Projects, AstraZeneca
Breaking Down Barriers: Streamlining CDAs to Accelerate Clinical Research with Javara, Regeneron, IQVIA, and Moffitt Cancer Center
Tuesday, February 3 at 3:30 pm
Panel Moderator: Christine Senn, Senior Vice President, Site-Sponsor Innovation, Advarra
- Jennifer Byrne, CEO, Javara, Inc.
- Patrick A. Floody, Vice President, Global Clinical Trial Services, Regeneron Pharmaceuticals, Inc.
- Alison Liddy, Senior Vice President, Patient and Site Centric Solutions, IQVIA
- Kristie Moffett, Senior Director, Moffitt Cancer Center
Study start-up delays remain a major barrier to clinical trial efficiency and patient access. The Site-Sponsor Consortium (SSC)—a collaboration among sponsors, CROs, and institutional and commercial sites—was formed to address these persistent challenges. This panel will unveil SSC’s inaugural whitepaper, outlining actionable strategies to streamline and harmonize CDA processes. Panelists will explore ethical and operational imperatives for reform, the role of master and bilateral CDAs, and the collaborative journey to consensus. Attendees will gain practical insights to accelerate start-up timelines and promote transparency, shared responsibility, and industry-wide alignment. Study start-up delays remain a major barrier to clinical trial efficiency and patient access. The Site-Sponsor Consortium (SSC)—a collaboration among sponsors, CROs, and institutional and commercial sites—was formed to address these persistent challenges. This panel will unveil SSC’s inaugural whitepaper, outlining actionable strategies to streamline and harmonize CDA processes. Panelists will explore ethical and operational imperatives for reform, the role of master and bilateral CDAs, and the collaborative journey to consensus. Attendees will gain practical insights to accelerate start-up timelines and promote transparency, shared responsibility, and industry-wide alignment.
Meeting Sites Where They Are—Key Connections in Action with Moderna, Immunovant, and Velocity Clinical Research
Wednesday, February 4 at 9:50 am
Speakers:- Adrienne Dunn, Associate Director of Clinical Operations, Moderna
- Nick Spittal, Chief Operations Officer, Velocity Clinical Research
- Reb Buckley, Director of Site Technology, Immunovant
- Kate Yawman, Director, Product Management, Advarra
Accelerating study start-up and improving operational outcomes requires sponsors to support sites within their established ways of working. This session explores how connected and easily accessible technology with aligned workflows can reduce inefficiencies, strengthen collaboration, and enhance visibility across study operations. Through real-world examples, we’ll highlight how greater technological harmony between stakeholders contributes to more efficient start-up, stronger engagement, and the realization of connected research.
Meet Our Speakers

Ian Bailey
Managing Director, AI & Data Science Analytics

Ashley Davidson
VP, Product Lead

Christine Senn

Kate Yawman
Director, Product Management
Where You’ll Find Us
Catch Us on the Course
We’re excited to support the 5th Annual Masters of Clinical Research Golf Tournament as the event’s Gold Golf Ball and Hole Sponsor. Swing by our sponsored hole to meet the team, pick up some swag, and take part in an event that gives back to CureSMA.
Take Home an Event T-Shirt
Don’t leave SCOPE without one of this year’s custom shirts. You can grab yours in the Keynote room or swing by Booth 701 during the event. Quantities are limited, so get yours while they last.
Recharge with Advarra
Take a break and power up at our exhibit hall charging area, featuring comfortable seating and easy access to outlets for all your devices.

BOOTH 701
Swing by to say hello to the Advarra team
- Stop by our booth to connect with our team and explore solutions that drive clinical research success.